The cement industry is responsible for more than 8% of the global CO2 emissions, making it one of the largest contributors to global warming. Two-thirds of these emissions are coming from unavoidable process gases caused by the calcination reaction of limestone (CaCO3). The remaining emissions are mainly coming from the combustion of fossil fuels. Due to the high temperature requirement (≈1500°C), direct electrification is difficult to consider. For these two reasons, the cement industry is therefore known as a challenging sector to decarbonize.
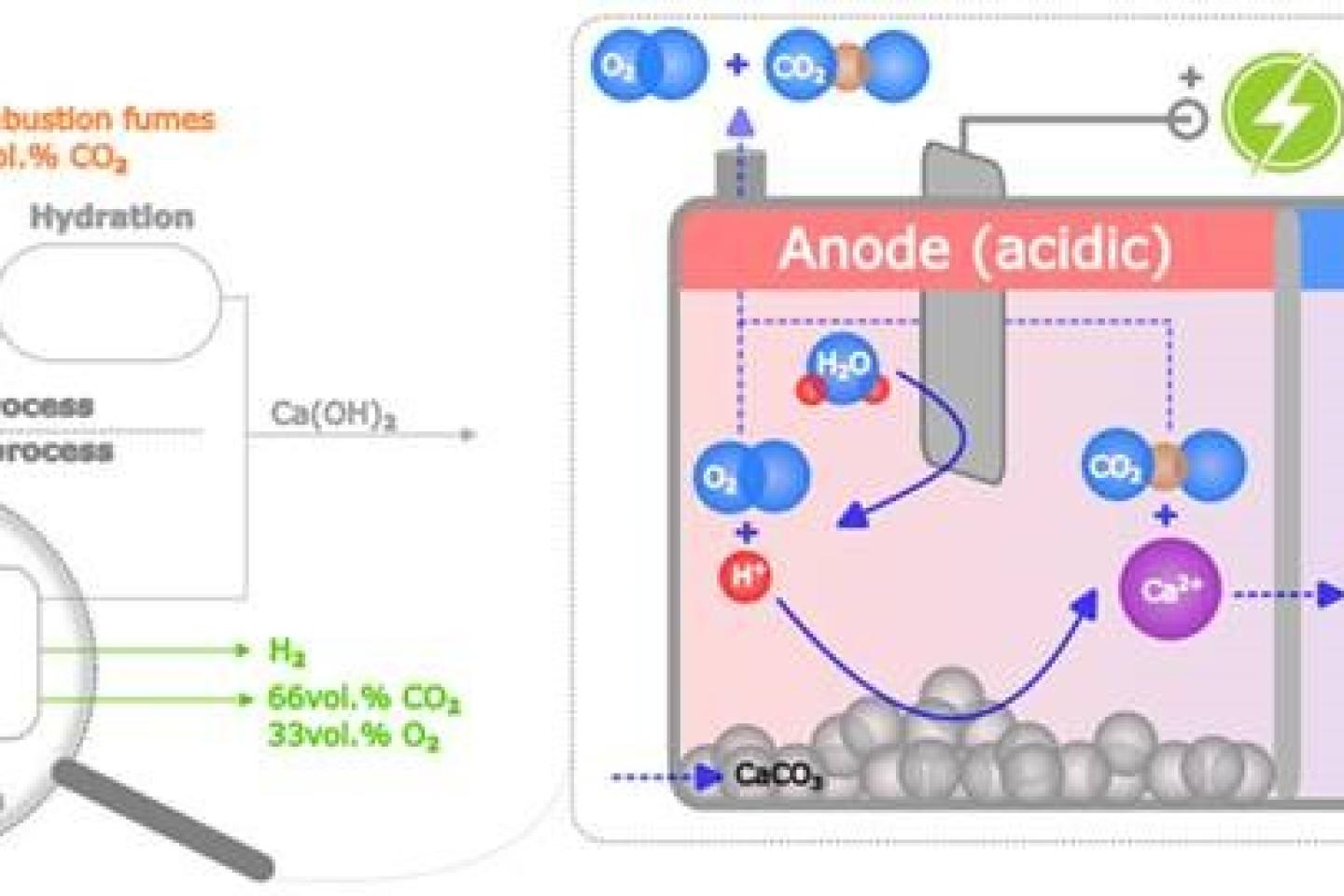
A new electrochemical pathway for cement production has gained prominence in the scientific literature in recent years [1]. This technology has the potential to electrify the cement and lime production while producing at the same time valuable gases (O2, CO2, H2). It is based on a new type of hybrid electrolyser capable of working with solid, liquid and gaseous phases. Thanks to the acidity generated by water oxidation at the anode, the solid limestone feed, precursor of cement, is being dissolved. This dissolution produces pure CO2  which is mixed with pure O2 generated by electrolysis and which is easily captured for later use. The calcium ions are then migrating towards the cathode under the effect of the electric field. The OH- hydroxyl ions, produced at the cathode by water reduction, meet the calcium ions to form the final solid product, hydrated lime (Ca(OH)2). This lime can be used as such or converted into cement by additional steps.
which is mixed with pure O2 generated by electrolysis and which is easily captured for later use. The calcium ions are then migrating towards the cathode under the effect of the electric field. The OH- hydroxyl ions, produced at the cathode by water reduction, meet the calcium ions to form the final solid product, hydrated lime (Ca(OH)2). This lime can be used as such or converted into cement by additional steps.
Speaker : Maxime Loudeche graduated at UCLouvain last year and is now working on the Faraday Project under the supervision of Prof. Joris Proost.

 which is mixed with pure O2 generated by electrolysis and which is easily captured for later use. The calcium ions are then migrating towards the cathode under the effect of the electric field. The OH- hydroxyl ions, produced at the cathode by water reduction, meet the calcium ions to form the final solid product, hydrated lime (Ca(OH)2). This lime can be used as such or converted into cement by additional steps.
which is mixed with pure O2 generated by electrolysis and which is easily captured for later use. The calcium ions are then migrating towards the cathode under the effect of the electric field. The OH- hydroxyl ions, produced at the cathode by water reduction, meet the calcium ions to form the final solid product, hydrated lime (Ca(OH)2). This lime can be used as such or converted into cement by additional steps.